Abstract
Background: Outcomes for patients with ultra-high risk (UHiR) newly diagnosed multiple myeloma (NDMM) and patients with primary plasma cell leukemia (pPCL) remain unsatisfactory with current standard therapies. Traditional comparative trials randomising against a standard of care control arm are thus challenging for patients with UHiR NDMM or pPCL, and novel approaches to address their high unmet need are required. OPTIMUM/MUKnine (NCT03188172) is a 'digital comparator arm' trial for UHiR NDMM and pPCL patients with protocol defined outcome comparison against fully molecularly matched UHiR patients from the near-concurrent NCRI Myeloma XI/XI+ trial, the 'MyXI prior'. We report final analysis of the primary endpoint progression free survival (PFS) at 18 months for patients treated in OPTIMUM with Dara-CVRd induction, V-augmented ASCT and Dara-VRd consolidation, compared to the MyXI prior.
Methods: Between Sep 2017 and Jul 2019, 472 patients from 39 UK hospitals with suspected NDMM or pPCL were screened. 107 patients with UHiR NDMM by central trial genetic (≥2 high risk lesions: t(4;14), t(14;16), t(14;20), gain(1q), del(1p), del(17p)) or gene expression SKY92 (SkylineDx) profiling, or with pPCL (circulating plasmablasts >20%) were identified and recruited to OPTIMUM. Patients received up to 6 cycles of Dara-CVRd induction, V-ASCT, followed by Dara-VRd consolidation 1 for 6 cycles (Cons1), Dara-VR consolidation 2 for 12 cycles and monthly Dara-R maintenance until progression. This is the final analysis of the primary trial endpoint progression-free survival (PFS) at 18 months comparing OPTIMUM with the MyXI prior of patients treated with CRd or carfilzomib-CRd (KCRd) induction, ASCT and R maintenance or observation, using a Bayesian framework. Secondary endpoints include PFS, OS, safety and quality of life.
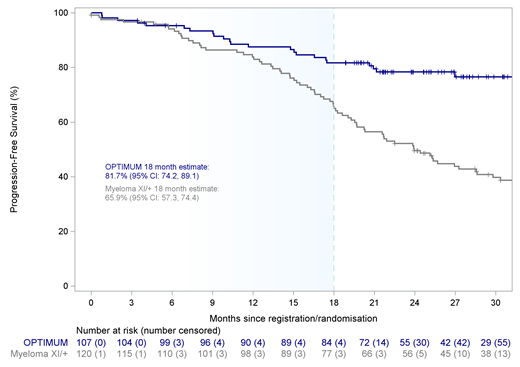
Results: At median follow-up of 27.1 months (95% CI 25.1-29.3), median PFS was not reached for OPTIMUM patients. PFS was superior at the pre-specified time point of 18 months for OPTIMUM patients with an estimate of 81.7% (95% CI: 74.2-89.1) versus 65.9% (95% CI: 57.3-74.4) for the MyXI prior (Figure 1). PFS at 18 months was consistently shorter for both CRd (64.5%; 95% CI: 53.8-75.3) and KCRd (68.3%; 95% CI: 54.0-82.5) treated patients compared with OPTIMUM. There was a 99.5% chance of superior PFS outcome with OPTIMUM therapy compared to the MyXI prior within the Bayesian framework; easily surpassing the 85% pre-specified threshold of sufficient evidence of activity. The difference between trial treatments increased over time: 6 month estimates were similar across all treatment arms with OPTIMUM 95.3% (95% CI: 91.3-99.3), MyXI KCRd 95.1% (95% CI: 88.5-100.0), MyXI CRd 93.5% (95% CI: 88.0-99.0), while 12 month estimates were similar for OPTIMUM with 87.5% (95% CI: 81.2-93.9) and MyXI KCRd 87.8% (77.8-97.8), but lower in CRd 81.7% (95% CI: 73.0-90.3). The majority (94%) of patients who started OPTIMUM Cons1 completed all 6 cycles of therapy. Most frequent grade 3/4 adverse events (AEs) during Cons1 included thrombocytopenia (27.9%), neutropenia (21%) and infection (19.8%), however, grade 4 events were rare (<5%) for all categories, consistent with previously presented data on induction. We previously reported high MRD-negativity rates of 61% at day +100 post V-ASCT for OPTIMUM, with a lower rate of 40% of patients showing both complete response (CR) and MRD-neg. With further follow-up, CR rate increased to 68.2% (95% CI: 58.5-76.9) at end of Cons1, including virtually all patients with MRD-neg finding post V-ASCT.
Conclusions: OPTIMUM demonstrated a clear PFS benefit at 18 months for intensified Dara combination therapy pre- and post-ASCT for UHiR NDMM and pPCL over the MyXI prior. Improvement of comparative benefit over time suggests particular efficacy of Dara-VRd in maintaining responses post ASCT, a key challenge in UHiR MM. This is, to our knowledge, the first prospective digital comparator trial for MM; central screening of an all-comer population combined with robust, detailed molecular matching maintained reliability and limited biases. These results demonstrate a novel framework for accelerated comparative evidence generation for patients with high unmet clinical need.
Kaiser: BMS/Celgene: Consultancy, Other: Travel support, Research Funding; Janssen: Consultancy, Other: Educational support, Research Funding; GSK: Consultancy; Karyopharm: Consultancy, Research Funding; Pfizer: Consultancy; Amgen: Honoraria; Seattle Genetics: Consultancy; Takeda: Consultancy, Other: Educational support; AbbVie: Consultancy. Hall: Janssen: Research Funding; BMS/Celgene: Research Funding. Garg: University Hospital Leicester: Current Employment; Takeda Janssen Novartis Sanofi: Other: Travel Accommodations, Expenses; Amgen Janssen Novartis Sanofi Takeda: Honoraria. Jackson: J and J: Consultancy, Honoraria, Speakers Bureau; GSK: Consultancy, Honoraria, Speakers Bureau; takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; amgen: Consultancy, Honoraria, Speakers Bureau; celgene BMS: Consultancy, Honoraria, Research Funding, Speakers Bureau; oncopeptides: Consultancy; Sanofi: Honoraria, Speakers Bureau. Cook: BMS/Celgene: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Sanofi: Consultancy; Karyopharm: Consultancy; Amgen: Consultancy. Pratt: Binding Site: Consultancy; Janssen: Consultancy; Takeda: Consultancy; Amgen: Consultancy; BMS/Celgene: Consultancy; Gilead: Consultancy. Drayson: Abingdon Health: Current holder of individual stocks in a privately-held company. Jenner: Janssen: Consultancy, Honoraria, Speakers Bureau; BMS/Celgene: Consultancy, Honoraria, Speakers Bureau; Pfizer: Consultancy; Takeda: Consultancy.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal